What Are DARZALEX FASPRO® and DARZALEX®?
DARZALEX FASPRO® and DARZALEX® are prescription medicines used alone or in combination with one or more treatments for adults with a rare form of blood cancer called multiple myeloma. See the indications below on this web page.
DARZALEX FASPRO® and DARZALEX® are not chemotherapies. They are targeted monoclonal antibodies that help slow or stop the progression of multiple myeloma in several ways.
Both treatments are made up of daratumumab (pronounced da-ra-tu-mu-mab ![]() ) to treat multiple myeloma. DARZALEX FASPRO® is also made up of hyaluronidase (pronounced hy-a-lur-on-i-dase
) to treat multiple myeloma. DARZALEX FASPRO® is also made up of hyaluronidase (pronounced hy-a-lur-on-i-dase ![]() ), which helps daratumumab to be injected into the skin and absorbed in the body.
), which helps daratumumab to be injected into the skin and absorbed in the body.
Explore the clinical trials for DARZALEX FASPRO® and DARZALEX®
How daratumumab works to fight multiple myeloma
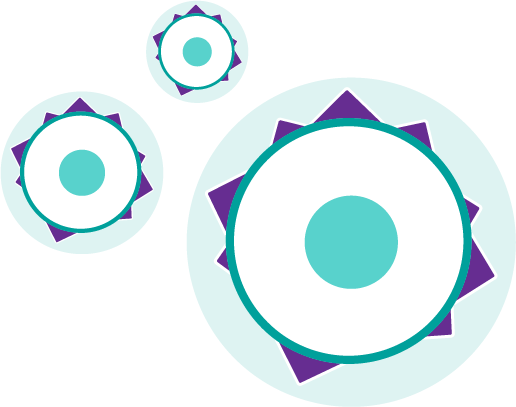
Multiple myeloma cells, like other types of cancer, can go unrecognized by your body's immune system, which allows the cells to grow.
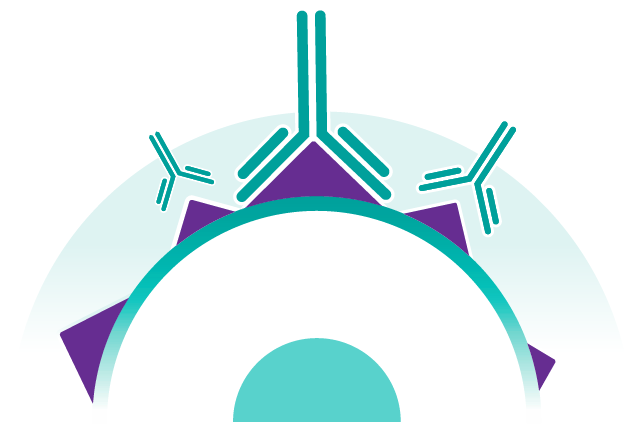
Daratumumab attaches itself to the CD38 protein on the surface of multiple myeloma cells, as well as on certain other types of cells, such as red blood cells.
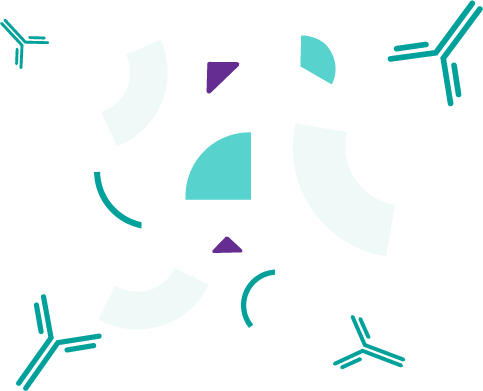
Daratumumab directly kills multiple myeloma cells and/or enables your immune system to identify and destroy them. Because of the way daratumumab works, it may also affect some normal cells.
Who are DARZALEX FASPRO® and DARZALEX® for?
Newly diagnosed
Transplant ineligible*
DRd: in combination with lenalidomide (R) and dexamethasone (d)
DVMP: in combination with bortezomib (V), melphalan (M), and prednisone (P)
Transplant eligible*
DVTd: in combination with bortezomib (V), thalidomide (T), and dexamethasone (d)
Previously treated
After ≥1 prior medicine
DRd: in combination with lenalidomide (R) and dexamethasone (d)
DVd: in combination with bortezomib (V) and dexamethasone (d)
DPd: in combination with pomalidomide (P) and dexamethasone (d) in patients previously treated with lenalidomide (R) and a PI
DKd: in combination with carfilzomib (K) and dexamethasone (d)
After ≥3 prior medicines
Alone: prior medicines included a PI and an immunomodulatory agent, or patients who did not respond to a PI and an immunomodulatory agent
*Newly diagnosed patients who can or cannot receive a type of stem cell transplant that uses their own stem cells.
D=DARZALEX FASPRO® or DARZALEX®.
