Additional clinical studies
Clinical studies for people with newly diagnosed multiple myeloma
The main goal of the study was to measure the length of time people lived without having their multiple myeloma get worse, or passing away from any cause. Another goal was to measure overall response rate, which is the percentage of people who responded to treatment.
706
people total participated in the study

350
people were treated with DARZALEX® + bortezomib + melphalan + prednisone (DVMP)
people were treated with DARZALEX® + bortezomib + melphalan + prednisone (DVMP)
356
people were treated with VMP alone
people were treated with VMP alone
People studied had newly diagnosed multiple myeloma and could not receive a type of stem cell transplant that uses their own stem cells.
At a median follow-up of 16.5 months
75%
(262 out of 350) of people in the DVMP group lived without having their disease get worse, or passing away
vs 60% (213 out of 356) of people in the VMP group.
About 9 out of 10 people responded to DARZALEX® + VMP vs about 7 out of 10 people treated with VMP alone.
42.6%
of people had a complete response* or better with DARZALEX® + VMP
vs 24.4% with VMP alone.
*This means the doctor observed no signs or symptoms of the disease as seen through imaging or other specific blood and bone marrow tests after treatment.
A study confirmed the effectiveness of DARZALEX FASPRO® when used in combination with bortezomib + melphalan + prednisone (VMP) in people with newly diagnosed multiple myeloma who could not receive a type of stem cell transplant that uses their own stem cells.
The main goal of the study was to measure overall response rate, which is the percentage of people who responded to treatment.
67
people participated in the study.
Everyone who participated was newly diagnosed with multiple myeloma and were unable to have a transplant using their own stem cells. They were treated with DARZALEX FASPRO® + bortezomib + melphalan + prednisone (DVMP)
At a median follow-up of 6.9 months
88%
(~9/10) of people responded to treatment with DARZALEX FASPRO® in combination with bortezomib, melphalan, and prednisone (DVMP)
DARZALEX® was studied in 1,085 people in combination with bortezomib + thalidomide + dexamethasone (VTd) vs VTd alone.
- People studied were newly diagnosed with multiple myeloma and were eligible to receive a type of stem cell transplant that used their own stem cells
- In this study, people received initial (induction) therapy with either DARZALEX® + VTd or VTd alone
- After induction, people received high-dose chemotherapy and a type of stem cell transplant that used their own stem cells
- People received consolidation therapy with either DARZALEX® + VTd or VTd alone. Consolidation therapy is given to help kill any cancer cells that may be left in the body after initial therapy and transplant
The main goal of the study was to measure stringent complete response, also known as sCR, which is a robust measure reflecting deep responses. Another goal of the study was to measure the length of time people lived without their multiple myeloma getting worse or passing away.
1,085
people total participated in the study

543
people were treated with DARZALEX® + bortezomib + thalidomide + dexamethasone (DVTd)
people were treated with DARZALEX® + bortezomib + thalidomide + dexamethasone (DVTd)
542
people were treated with VTd alone
people were treated with VTd alone
Clinical study results
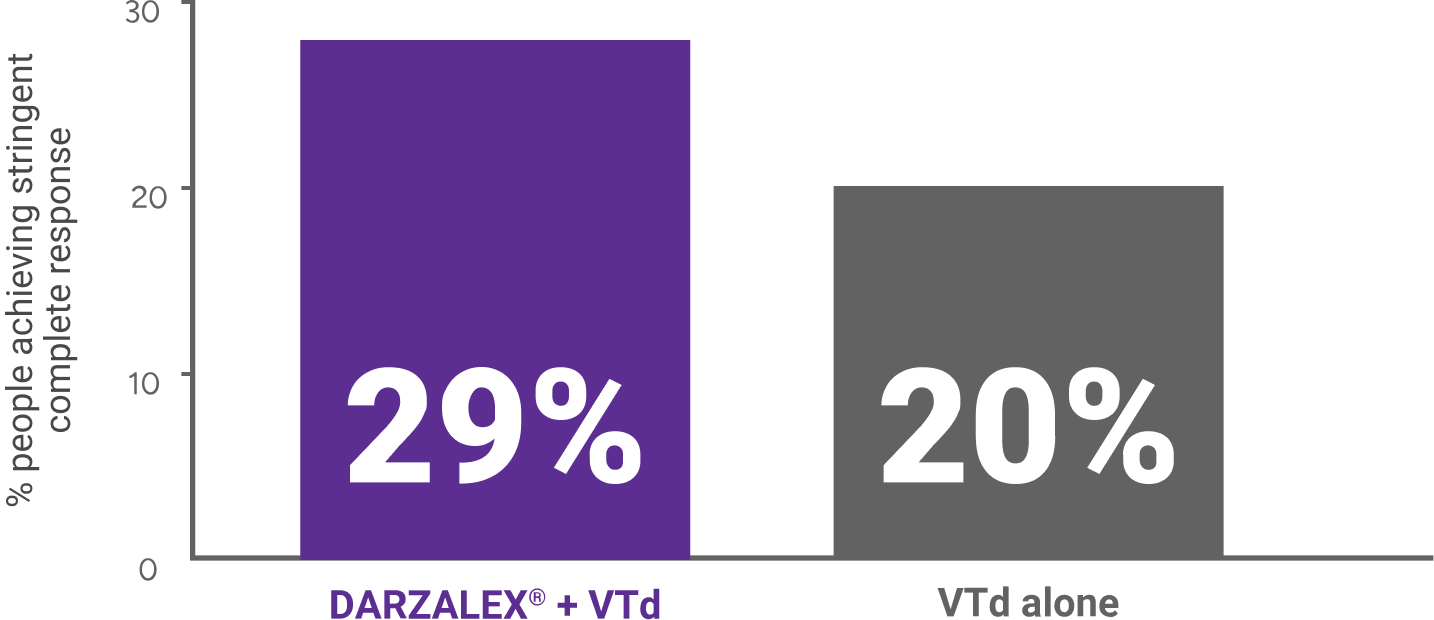
More people achieved sCR with DARZALEX® in combination with VTd compared with people treated with VTd alone. 29% of people treated with DARZALEX® + VTd (n=543) achieved sCR vs 20% of people treated with VTd (n=542) alone.
The overall response rate (which includes all levels of response) was 93% of people treated with DARZALEX® + VTd vs 90% of people treated with VTd alone.
At a median follow-up of 18.8 months
91.7%
(498 out of 543) of people in the DVTd group lived without having their disease get worse, or passing away
vs 83.2% (451 out of 542) of people in the VTd group.
~90% of people responded to DARZALEX® + VTd vs ~90% of people treated with VTd alone.
- 38.8% of people had a complete response* or better with DARZALEX® + VTd vs 26% with VTd alone
*This means the doctor observed no signs or symptoms of the disease as seen through imaging or other specific blood and bone marrow tests after treatment.
Studies for relapsed or refractory multiple myeloma
A study confirmed the effectiveness of DARZALEX FASPRO® when used in combination with lenalidomide + dexamethasone (Rd) for relapsed or refractory multiple myeloma.
The main goal of the study was to measure overall response rate, which is the percentage of people who responded to treatment.
65
people participated in the study.
Everyone who participated was diagnosed with multiple myeloma and had received at least one prior medicine.
91%
(~9/10) of people responded to treatment with DARZALEX FASPRO® in combination with lenalidomide and dexamethasone (DRd).
The main goal of the study was to measure the length of time people lived without having their multiple myeloma get worse, or passing away. Another goal was to measure overall response rate, which is the percentage of people who responded to treatment.
569
people total participated in the study

286
people were treated with DARZALEX® + lenalidomide (R) + dexamethasone (d)
people were treated with DARZALEX® + lenalidomide (R) + dexamethasone (d)
283
people were treated with Rd alone
people were treated with Rd alone
People studied had received at least one prior medicine to treat their multiple myeloma.
At a median follow-up of 13.5 months
82%
(233 out of 286) of people in the DRd group lived without having their disease get worse, or passing away
vs 59% (167 out of 283) of people in the Rd group.
About 9 out of 10 people responded to the IV formulation of DARZALEX® + Rd vs about 7 out of 10 people treated with Rd alone.
- 42.3% of people had a complete response* or better with DARZALEX® + Rd vs 18.8% with Rd alone
*This means the doctor observed no signs or symptoms of the disease as seen through imaging or other specific blood and bone marrow tests after treatment.
The main goal of the study was to measure the length of time people lived without having their multiple myeloma get worse, or passing away. Another goal was to measure overall response rate, which is the percentage of people who responded to treatment.
498
people total participated in the study

251
people were treated with DARZALEX® +
bortezomib + dexamethasone (DVd)
247
people were treated with Vd alone
people were treated with Vd alone
People studied had at least one prior medicine to treat their multiple myeloma.
At a median follow-up of 7.4 months
73%
(184 out of 251) of people in the DVd group lived without having their disease get worse, or passing away
vs 51% (125 out of 247) of people in the Vd group.
About 8 out of 10 people responded to DARZALEX® + Vd vs 6 out of 10 people treated with Vd alone.
- 18.3% of people had a complete response* or better with DARZALEX®+ Vd vs 8.5% with Vd alone
*This means the doctor observed no signs or symptoms of the disease as seen through imaging or other specific blood and bone marrow tests after treatment.
A study confirmed the effectiveness of DARZALEX FASPRO® when used in combination with pomalidomide + dexamethasone (Pd) for relapsed or refractory multiple myeloma.
The main goal of the study was to measure the length of time people lived without having their multiple myeloma get worse, or passing away.
304
people total participated in the study

151
people were treated with DARZALEX FASPRO® + pomalidomide + dexamethasone (DPd)
153
people were treated with Pd alone
people were treated with Pd alone
People studied had at least one prior medicine and were previously treated with lenalidomide (R) and a proteasome inhibitor (PI).
At a follow-up of 18 months
44.3%
(67 out of 151) of people in the DPd group lived without having their disease get worse, or passing away
vs 30.7% (47 out of 153) of people in the Pd group.
DARZALEX® was studied in combination with pomalidomide + dexamethasone (Pd) in 103 people who had received a prior proteasome inhibitor (PI) and an immunomodulatory agent. These people had received a median of 4 prior lines of therapy for their multiple myeloma.
The main goal of the study was to measure overall response rate, which is the percentage of people who responded to treatment.
Clinical study results
When these people were treated with DARZALEX® in combination with Pd, 59% responded.
- This response lasted for a median duration of 13.6 months (range: 0.9+ to 14.6+ months)
Of people who responded, response was seen between 0.9 and 2.8 months.
1 month: Median time it took people to respond to DARZALEX® + Pd.
DARZALEX® was studied as monotherapy (by itself) in 106 people who had either received at least 3 prior medicines to treat their multiple myeloma, including a proteasome inhibitor (PI) and an immunomodulatory agent, or did not respond to a PI and an immunomodulatory agent. These people had received a median of 5 prior lines of therapy for their multiple myeloma.
The main goal of the study was to measure overall response rate, which is the percentage of people who responded to treatment.
Clinical study results
When these people were treated with DARZALEX®, 29% responded.
- This response lasted for a median duration of 7.4 months (range: 1.2 to 13.1+ months)
Of people who responded, response was seen between 0.9 and 5.6 months.
1 month: Median time it took people to respond to DARZALEX®.
DARZALEX FASPRO® and DARZALEX® comparison study
In order to understand how DARZALEX FASPRO® compared to DARZALEX®, DARZALEX FASPRO® was evaluated as monotherapy (alone).
A study confirmed that DARZALEX FASPRO® gave people results comparable to DARZALEX® in treating multiple myeloma when used by itself.
This study compared treatments in people with multiple myeloma who had received at least 3 prior medicines or who did not respond to a proteasome inhibitor (PI) and an immunomodulatory agent.
The main goal of the study was to measure overall response rate, which is the percentage of people who responded to treatment.
522
people total participated
in the study

263
people were treated with
DARZALEX FASPRO®
259
people were treated with
DARZALEX®
41%
(~4/10) of people responded to treatment with DARZALEX FASPRO®
vs 37% (~4/10) who responded to treatment with DARZALEX®.
In the clinical study the results were consistent, with a similar number of people responding to both treatments.